The Long-Term Progression Free Survival After the Combination of Radiotherapy with Concurrent Chemotherapy of Nimotuzumab And Temozolomide Followed Adjuvant Temozolomide In Recurrent Anaplastic Astrocytoma-Juniper Publishers
Global Journal of Reproductive
Medicine Juniper
Publishers
Authored by: Xiaoqi Xie*Introduction
Gliomas which originated from glial cells and made up 80% of all primary CNS tumors in the United States were classified as “low grade” (WHO grades I and II) and “high grade” (WHO grades III and IV) according to histopathologic changes [1,2]. High-grade gliomas (HGGs) are related with poor prognosis and consist of anaplastic gliomas (anaplastic astrocytoma, anaplastic oligodendroglioma and anaplastic oligoastrocytoma; WHO grade III) and glioblastomas (GBMs; WHO grade IV). The current standard therapy for newly diagnosed GBMs involves maximal safe resection followed by radiotherapy (RT) with concomitant and adjuvant temozolomide (TMZ) [3-5]. Despite optimal therapy, the median time-to-tumor progression (mTTP) for patients with newly diagnosed GBMs is approximately 6.9 months and these tumors unavoidably relapse with a median overall survival (mOS) of only 15 months [3]. However, the treatment of anaplastic gliomas is abroad controversial topics in neuro-oncology. By above therapy, anaplastic gliomas achieves a mTTP of about 1.2 to 2.6 years and a mOS of 2 to 7 years [4,6,7].
Compared with anaplastic oligodendroglioma, treatment of the anaplastic astrocytoma has been less optimistic. This tumor is more resistant to therapy and patients have a shorter mOS of only 2 to 3 years. In the United States, most oncologists treat anaplastic astrocytoma patients with maximal safe resection followed by field radiotherapy with concurrent and adjuvant chemotherapy (temozolomide), same to the regimen now deemed as the standard of care for GBMs [3]. Moreover, all HGGs nearly recur and survival following disease progression is doomed to be approximately 6 months for GBMs and 10 months for anaplastic gliomas [8,9]. Treatment options for recurrent HGGs include re-resection, chemotherapy, or re-irradiation. But, the optimal treatment strategies for recurrent HGGs are still unknown and the randomized control trials which contrast active intervention and determine standard therapy are absent. We herein report the case of a recurrent anaplastic astrocytoma patient with the long-term progression free survival (PFS) after the combination of radiation therapy with concurrent chemotherapy of nimotuzumab and temozolomide followed adjuvant temozolomide.
Case Report
We present the unique case of a 33-year-old gentleman who initially presented with a several days history of headaches behind the right brain, with associated “vomiting”. There were no complaints of numbness, weakness, or any visual changes. Physical examination of the patient revealed that he had a depressed superficial reflex in the right limbs and positive pyramidal tract signs. A MRI scan at that time revealed a large, irregularly enhancing mass in the right frontal lobe that was associated with marked edema, bleeding and mass effect. These symptoms relieved with the treatment of dehydration to reduce intracranial pressure. Following the consultation with a neurosurgeon and discussing the probability of a brain tumor and therapeutic regimen, the patient experienced a right frontal lobe Lobectomy in local hospital in October 2008. The final pathology was anaplastic astrocytoma (WHO grade III).
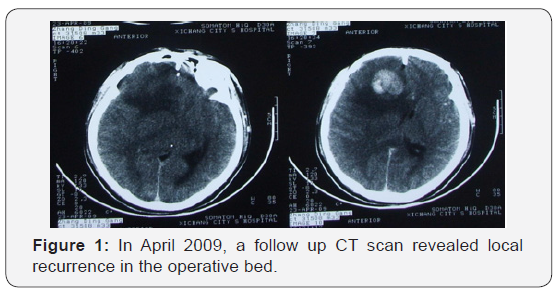
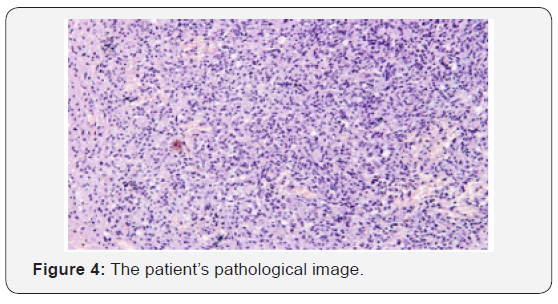
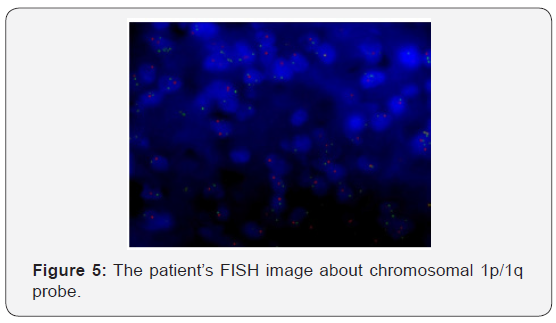
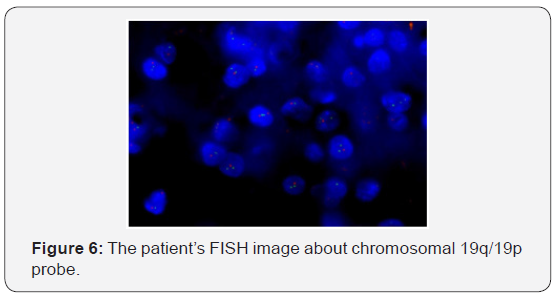
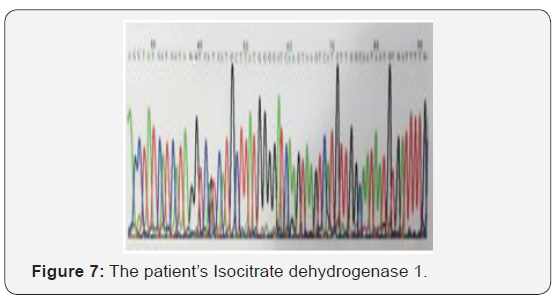
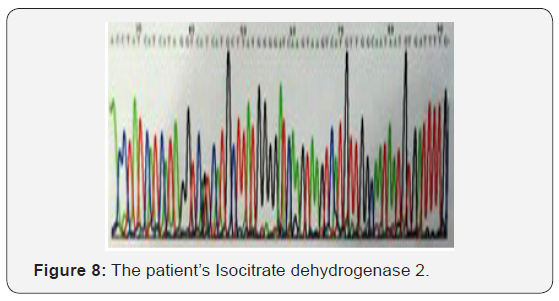
The patient recovered well from surgery, and his headaches were resolved. Adjuvant treatment options were explained to him and included radiotherapy plus concomitant and adjuvant Temozolomide. But the patient refused further therapy. In April 2009, a follow-up CT scan revealed local recurrence in the operative bed (Figures 1-3). And the patient was treated in West China Hospital. The pathology department in West China Hospital held a pathological consultation with tumor section in local hospital and diagnosed anaplastic astrocytoma (WHO grade III) containing a few of oligodendroglioma ingredients with histopathologic changes of partial necrosis, mitotic activity and endothelial cell proliferation (Figure 4). KRAS mutation was detected in codon 12 and codon 13 and the DNA repair enzyme O6-methylguanine- DNA methyltransferase (MGMT) promoter methylation was positive. Chromosomal 1p and 19q co-deletion was detected by FISH (Figures 5&6). Isocitrate dehydrogenase 1 mutation (codon 132) was detected by PCR and Sanger sequencing and Isocitrate dehydrogenase 2 (codon 172) was wild type (Figures 7&8).
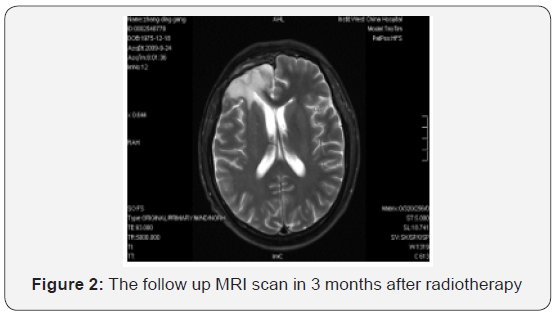
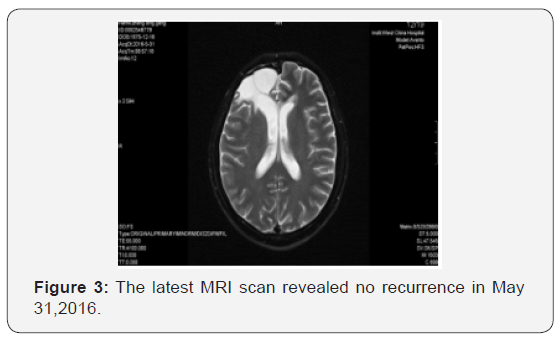
Starting on May 22, 2009, the patient underwent whole brain radiotherapy to a dose of 4000 cGy and local lesion radiotherapy to a dose of 6000 cGy (fractionated focal irradiation in daily fractions of 2 Gy given 5 days per week for 6 weeks) with concurrent combined chemotherapy of intravenous nimotuzumab (100 mg every 1 weeks) and oral temozolomide (75 mg per square meter of body-surface area per day) for 6 weeks followed by six cycles of adjuvant temozolomide (150 mg per square meter for 5 days during each 28-day cycle). Treatment was generally well tolerated, and the adverse events were nausea, vomiting and hematologic toxicities, which all of them were classified as mild. Following his radiotherapy and chemotherapy, he was followed clinically as well as with surveillance MRI scans at regular intervals. The follow-up MRI scan in 3 months after radiotherapy were as follows (Figure 2). The latest MRI scan revealed no recurrence in May 31, 2016 (Figure 3). Until now, the patient is still alive with a long-term progression free survival lasting seven years. And the survival following the initial diagnosis is up to eight years.
Discussion
A high rate of local recurrences has been observed after multi-disciplinary therapy in high-grade gliomas (HGGs) patients.10 Important variables predicting longer survival include oligodendroglial cell line, extent of resection, and younger age (<50 years) [11]. Moreover, gliomas with the DNA repair enzyme O-6-methylguanine-DNA methyltransferase (MGMT) promoter methylation, chromosomal 1p and 19q co-deletion or isocitrate dehydrogenase 1 and 2 (IDH1/2) mutations is associated with a better prognosis [12-15]. In this patient, the chromosomal 1p and 19q co-deletion was detected by FISH; and the MGMT promoter methylation was positive and Isocitrate dehydrogenase 1 was mutation type, which was one of the reason of long-term progression free survival (PFS). PFS is associated with overall survival (OS) and has become a marker for evaluating the efficacy of treatments in recurrent HGGs patients. The 6-month PFS (PFS6) for recurrent GBM ranges from 9 to 21% [8,9,16], while PFS6 for anaplastic gliomas have ranged from 37 to 48% [17,18]. In our report, the patient still does not progress with a long-term PFS lasting seven years. Although the prognosis of recurrence HGGs patients is invariably poor, it is vital just as this case to improve the quality of life and prolong survival by multimodal therapy.
Therapy options for recurrent HGGs are limited and may include surgery, re-irradiation, chemotherapy and targeted therapies. For recurrent HGGs patients which were subjected to clinical deterioration on account of mass effect, surgery might be one of option. But, until now, no prospective researches demonstrate that re-surgery can improve the survival for recurrent HGGs patients. Moreover, due to the application of targeted therapies, a fewer proportion of recurrent HGGs patients are going through diagnostic or therapeutic re-surgery.
For HGGs patients, primary therapy most involves adjuvant radiotherapy with the maximum tolerated dose. The dose limitation of healthy brain tissue and the short duration between initial radiation therapy and recurrence confined the use of reirradiation therapy for recurrent HGGs with conventional external beam radiotherapy, which may have an influence on patient’s quality of life. However, Romanelli et al’ s research that evaluated the efficacy of stereotactic radiosurgery (SRS) in recurrent glioma have indicated median OS from 7.5 to 30 months after treatment [19]. And in Mahajan’s research [20], recurrent GBM patients treated with SRS achieved a longer median OS compared with untreated patients [20]. Hypofractionated stereotactic irradiation might be another choice for recurrent glioma. According to Fogh, et al. [21] research, the median OS after re-irradiation with a hypofractionated protocol (3.5 Gy per fraction up to a median dose of 35 Gy) over a 2-week period was 11 months. In this case, because patient did not receive any further treatment after surgery, conventional external beam radiotherapy (whole brain radiotherapy to a dose of 4000 cGy and local lesion radiotherapy to a dose of 6000 cGy) plus concomitant and adjuvant Temozolomide were recommended to him and the progression free survival after irradiation was seven years.
The efficacy of chemotherapy in recurrent HGGs was limited. And many chemotherapeutic agents, including single agents and combination chemotherapy agents, have been used to treat recurrent HGGs [22,23]. Temozolomide, PCV and bevacizumab are some of the treatment choice according to several researches [24-27]. Temozolomide was evaluated in recurrent HGGs before becoming the first line chemotherapy for glioma. The recurrent anaplastic glioma patients that were treated with single-agent TMZ showed a 35% response rate and PFS6 of 46% [28]. Bevacizumab has been authorized by FDA as the treatment of recurrent GBMs [29]. Moreover, Nimotuzumab is a humanized monoclonal antibody which binds the extracellular domain of epidermal growth factor receptor (EGFR) and blocks EGFR signal pathway activation [30]. And the efficacy of nimotuzumab in HGGs is depended on the combination of nimotuzumab with radiochemotherapy or radiotherapy. Solomon et al. [31] research has illustrated the median survival time of the combination of nimotuzumab with radiotherapy was 12.4 months or 27.0 months for patients with GBM or anaplastic astrocytoma patients. In our case, the chemotherapy agents were the concurrent combined chemotherapy of intravenous nimotuzumab and oral temozolomide for 6 weeks followed by six cycles of adjuvant temozolomide. And the efficacy was surprising. Thus, nimotuzumab might be one of the chemotherapy choice and more randomized clinical trials need to be done to clarify the efficacy of nimotuzumab.
Conclusion
This is the first reported case to our knowledge of a patient with recurrent anaplastic astrocytoma who achieved PFS lasting seven years following treatment with the combination of radiation therapy with temozolomide and nimotuzumab. The patient tolerated the therapy well and do not relapse again until now. This case highlights the unique biology of individual glioma and that the combination of radiation with chemotherapy and targeted therapy in the recurrent setting can have a significantly influence on overall survival for specific patients. We will continue doing research in this area, especially in the molecular characteristics of individual tumors. Individualized treatment according to each unique patient’s situation will improve survival as well as quality of life.


Comments
Post a Comment